Background: Sabatolimab (MBG453) is a novel immunotherapy targeting TIM-3, an immuno-myeloid regulator expressed on both immune and leukemic stem cells. In the randomized, double-blind, placebo-controlled, Phase (Ph) II STIMULUS-MDS1 study (NCT03946670) in patients (pts) with HR-MDS, although improvements in complete remission (CR) and progression-free survival (PFS) were not statistically significant, sabatolimab + HMA was associated with longer duration of response (DoR), and the data suggest a delayed-onset benefit in the sabatolimab arm, as well as a potential treatment effect in pts with lower disease burden (Zeidan AM, et al. ASH 2022). The clinical significance of MRD in HR-MDS is not well established. We report an exploratory analysis of mutational clearance and MRD by next-generation sequencing (NGS) to evaluate the association with clinical outcomes.
Methods: Treatment-naive pts aged ≥18 years with intermediate (+ ≥5% bone marrow [BM] blasts), high or very high risk MDS by Revised International Prognostic Scoring System were randomized to sabatolimab or placebo added to azacitidine/decitabine (Zeidan AM, et al. ASH 2022). Peripheral blood (PBMC) and/or BM mononuclear cell (BMMC) samples were collected and NGS performed on genomic DNA extracted from baseline (BL) and on-treatment samples using a 38-gene NGS-error-corrected panel with sensitivity of 2% and 0.2% variant allelic frequency (VAF), respectively. Concordance and correlation between PBMC and BMMC were assessed. The prognostic value of clonal clearance at different VAF cut-offs (0.2%, 0.5% or 1%) was assessed. MRD-x status was defined as any mutation call above VAF cut-off x, excluding mutations in DNMT3A, TET2 and ASXL1 ( DTA). Association between MRD status and clinical outcomes was analyzed regardless of treatment to increase sample and used best overall response, 6-month landmark analysis, and a time-dependent Cox model to rule out immortal bias.
Results: NGS was run on 332 BMMC samples from 112 pts and 439 PBMC samples from 123 pts (127 pts randomized). 112 pts had ≥1 qualifying BL mutation (NGS cohort) and 106 pts (sabatolimab + HMA, N=56; placebo + HMA, N=50) had ≥1 at follow-up (MRD cohort). BL characteristics were balanced between NGS/MRD cohorts and between treatments in the MRD cohort.
Overall agreement in mutation calling at variant level between BMMC and PBMC was >97% (8312/8566 total mutation calls), with >88% positive (817/926) and >98% negative agreement (7495/7640). Across reported mutated variants (N=817), there was good correlation (pearson cor=0.88) in VAF between BMMC and PBMC. When considering all paired samples, concordance of best MRD status using PBMC or BMMC results at the pt level (N=95) was: MRD-0.2, 97%; MRD-0.5, 89%; MRD-1, 90%. Given good PBMC and BMMC agreement, results were combined (higher VAF used if both) for outcomes analysis.
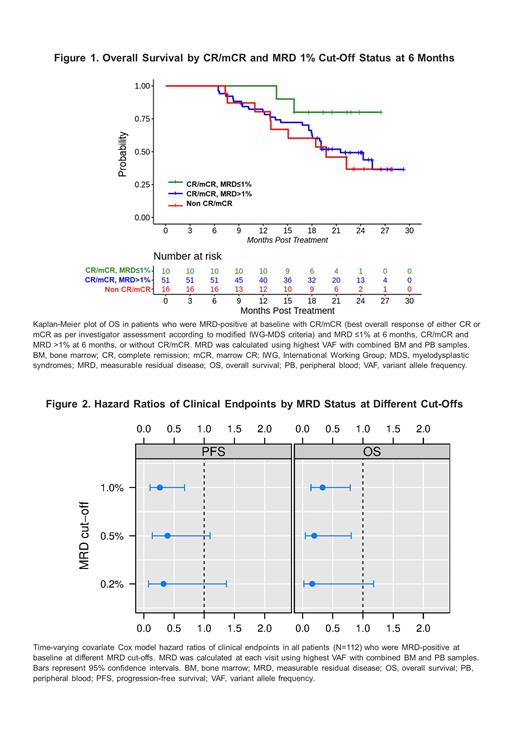
In the MRD cohort, 11% (N=12), 18% (N=19) and 27% (N=29) of pts achieved MRD-0.2, MRD-0.5 and MRD-1 negativity, respectively. Most pts with MRD-0.2, -0.5 and -1 clearance had documented response of CR/marrow CR [mCR] (>80%) or CR/partial remission [PR]/hematologic improvement [HI] (>75%). Best overall response of MRD-1 negativity achieved anytime on treatment was associated with improved PFS and overall survival (OS) in the overall population and for responding pts (CR/mCR or CR/PR/HI). Similar results were obtained in a landmark analysis at 6 months ( Fig 1). This was supported by a time-dependent Cox-model indicating that MRD-x negativity was associated with a lower hazard ratio for PFS and OS vs MRD-x positivity ( Fig 2).
A consistently higher proportion of pts in the sabatolimab (N=56) vs placebo (N=50) arms had on-treatment mutation clearance at different cut-offs: MRD-0.2, 16.1% vs 6.0%; MRD-0.5, 25.0% vs 10.0%; MRD-1, 35.7% vs 18.0%, respectively.
Conclusions: We present the first results from a prospective, controlled, randomized study demonstrating the potential prognostic value of MRD for PFS and OS in HR-MDS. Our results demonstrate high NGS concordance between PBMC and BMMC on-treatment samples, indicating that PBMC could represent an alternative for clonal monitoring. Interestingly, more pts treated with sabatolimab + HMA reached MRD negativity while in remission, potentially explaining the longer DoR in pts receiving sabatolimab + HMA vs placebo + HMA. These findings will be further explored in the ongoing Ph III STIMULUS-MDS2 study (NCT04266301).
Disclosures
Zeidan:Schrödinger: Consultancy, Honoraria; Notable: Consultancy, Honoraria; Chiesi: Consultancy, Honoraria; Mendus: Consultancy, Honoraria; Otsuka: Consultancy, Honoraria; Foran: Consultancy, Research Funding; Syros: Consultancy, Honoraria; ALX Oncology: Consultancy, Honoraria; Kura: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Boehringer-Ingelheim: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Orum: Consultancy, Honoraria; Geron: Consultancy, Honoraria; Syndax: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Zentalis: Consultancy, Honoraria; BeyondSpring: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Shattuck Labs: Research Funding; Agios: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Tyme: Consultancy, Honoraria; Taiho: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Ionis: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Astex: Research Funding; Lox Oncology: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; BioCryst: Consultancy, Honoraria; Regeneron: Consultancy, Honoraria. Fenaux:Novartis: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Jazz: Consultancy, Honoraria, Research Funding; French MDS Group: Honoraria; Janssen: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding. Han:Novartis: Current Employment. James:Novartis: Current Employment. Malek:Novartis: Current Employment. Marques Ramos:Novartis: Current Employment. Miyazaki:Novartis: Honoraria; Celgene: Honoraria; Dainippon-Sumitomo: Honoraria; Nipponshinnyaku: Honoraria; Chugai: Honoraria; Otsuka: Honoraria; Astellas: Honoraria; Kyowa-Kirin: Honoraria. Platzbecker:Geron: Consultancy, Research Funding; Janssen Biotech: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Curis: Consultancy, Research Funding; AbbVie: Consultancy; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; medical writing support, Research Funding; Amgen: Consultancy, Research Funding; Merck: Research Funding; Jazz: Consultancy, Honoraria, Research Funding; Syros: Consultancy, Honoraria, Research Funding; Servier: Consultancy, Honoraria, Research Funding; Silence Therapeutics: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Celgene: Honoraria; MDS Foundation: Membership on an entity's Board of Directors or advisory committees; Fibrogen: Research Funding; Roche: Research Funding; BeiGene: Research Funding; BMS: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal